Phoebe Stapleton, Ph.D., A.T.C.
Associate Professor Rutgers University-Ernest Mario School of PharmacyEOHSI- Toxicology
Biographical Info
Dr. Stapleton is an Assistant Professor in the Rutgers University, Ernest Mario School of Pharmacy, Department of Pharmacology and Toxicology, and the Joint Graduate Program in Toxicology. She received her B.S. in Biology and Athletic Training from State University of New York (SUNY) College at Cortland, a M.S.Ed. in Kinesiology from Southern Illinois University at Edwardsville, and a Ph.D. in Exercise Physiology from West Virginia University. She completed her postdoctoral training within the Department of Physiology and Pharmacology at West Virginia University.
Research Areas
The microcirculation branch of the cardiovascular system encompasses the arterioles, capillaries, and venules within an organ or tissue of interest. These highly active vessels serve to maintain homeostasis by regulating blood flow and tissue perfusion, thus providing nutrients and removing waste. Central to proper reactivity is the health and function of the endothelium, a single cell layer lining the vasculature. The Stapleton laboratory investigates the microvascular perturbations associated with normal physiological challenges (exercise or pregnancy), disease, and exposures to environmental and/or occupational xenobiotics.
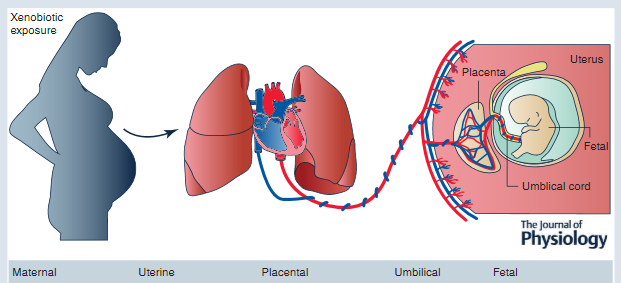
Using engineered nanomaterials, studies focus on the question: how can something we inhale affect the cardiovascular system? Recently, her research group has investigated non-traditional models of exposure by incorporating reproductive toxicology. These studies focus on exposures during pregnancy leading to the development of a hostile gestational environment identified through microvascular evaluations of the mother. These prenatal exposures impact fetal development and may predispose future generations to cardiovascular aberrations. The Stapleton laboratory is funded by a NIEHS ONES award, NIH R01 ES031285.
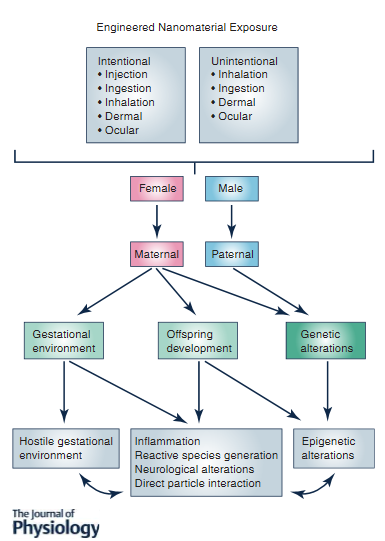
Research Highlights
- Identified and visualized nanoplastic particles are able to translocate from the maternal lungs to the fetal tissues after pulmonary exposure in late pregnancy.
- Development of a novel placental perfusion technique to quantify material transfer from the maternal uterine circulation to the fetal umbilical circulation and measure biomarkers of uteroplacental function.
- Identification of the development of a hostile gestational environment after engineered nanomaterial exposure during pregnancy.
- Development of a novel intravital microscopy technique to visualize the vasculature of the pregnant uterus.
- Investigations of mitochondrial function and bioenergetics in young exposed to xenobiotic matter during gestation.
- Identification of microvascular dysfunction and behavioral alterations associated with engineered nanomaterial exposure in gestation.
- Inhaled micro- and nanoplastic particles (MNP) aerosols were found to alter inflammatory, cardiovascular, and endocrine activity in virgin female Sprague-Dawley rats.
- Identified and visualized micro- and nanoplastic particles that have translocated from the maternal lungs or GI system to the fetal tissues 24h after pulmonary or gastric exposure, respectively, in late pregnancy.”
Awards
- Dr. Stapleton received the Outstanding New Environmental Scientist (ONES) Award from NIEHS.
- Dr. Stapleton was awarded the Society of Toxicology (SOT) Achievement Award (2024)
- Ms. Chelsea Cary’s manuscript (Single inhalation exposure to polyamide micro and nanoplastic particles impairs vascular dilation without generating pulmonary inflammation in virgin female Sprague Dawley rats) was awarded the Impact Award for the Cardiovascular Toxicology Specialty Section and the Best Manuscript Award for the Occupational and Public Health Specialty Section of SOT (2024)
- Laboratory publication, Ingested polystyrene micro-nanoplastics (MNPs) translate to placenta and fetal tissues in pregnant rats identified as top 1% of NIEHS papers published in 2023″
- Ms. Chelsea Cary awarded the Rutgers School of Graduate Studies Aaron Shatkin Graduate Award (2023)
- Ms. Talia Seymore was a finalist for the Trophoblast Research New Investigator Award, International Federation of Placental Association (IFPA) (2023)
- Ms. Chelsea Cary was awarded a Kirschstein-NRSA (F31) from NIEHS (2023)
- Ms. Chelsea Cary and Talia Seymore were each awarded a Diversity Initiatives Career Development Award from SOT to attend the IFPA meetings, individually (2023)
- Dr. Stapleton was awarded the Women in Toxicology (WIT) Outstanding Young Investigator award (2023)
- Laboratory publication, Maternal, placental, and fetal distribution of titanium after repeated titanium dioxide nanoparticle inhalation through pregnancy identified as top 1% of NIEHS papers published in 2022
- Mr. Andrés D. Rivera Ruiz was awarded the SOT Undergraduate Diversity Award in 2021 for his work as an 2020 Virtual SURF trainee.
- Ms. Chelsea Cary won the 2021 Graduate Student Trainee Award from the Cardiovascular Toxicology Specialty Section of the Society of Toxicology (SOT).
- Dr. Stapleton received the 2021 New Career Scientist Award from the Reproductive and Developmental Toxicology Specialty Section of the Society of Toxicology (SOT).
- Dr. Stapleton received the 2020 Young Investigator Award from the Inhalation and Respiratory Toxicology Specialty Section of the Society of Toxicology (SOT).
- Invited speaker to the NIEHS 50th Anniversary Celebration with SOT titled SOT and NIEHS Past, Present, and Future: 50 Years of Collaboration.
- Past-President of the Allegheny-Erie Regional Chapter of the Society of Toxicology.
- Dr. Stapleton recently published a symposia review of Gestational Nanomaterial Exposures in the Journal of Physiology (2016) 594(8):2161-73. (http://www.ncbi.nlm.nih.gov/pubmed/26332609)
- Dr. Stapleton has been awarded the Impact Award by the Cardiovascular Toxicology Specialty Section of SOT (2016), the Best Publication Award by the Nanotoxicology Specialty Section of SOT (2016), and the Best Postdoctoral Publication Award by the Postdoctoral Assembly of SOT (2014).
- Appointed as Review Editor for Frontiers in Vascular Physiology (2012).
In the News
- Interviewed for NBC News NOW, Live Broadcast (segment at 39:42), by Savannah Sellersand Joe Fryer Interviewed by WABC 7, “Is bottled water safe to drink? Expert weighs in on ‘nanoplastics’ and latest study”, by Josh Einiger
-
Interviewed by Corus National Radio (Canada) “A Little More Conversation”, “How much plastic are we ingesting when drinking bottled water”, by Ben O’Hara-Byrne
-
Interviewed by CNN, “Bottled water contains thousands of nanoplastics so small they can invade the body’s cells, study says,” by Sandee LaMotte
-
Interviewed for Associated Press, “Scientists find about a quarter million invisible nanoplastic particles in a liter of bottled water”, by Seth Borenstein
-
Interviewed by The Grist, “Bottled water has up to 100 times more plastic particles than previously thought”, by Joseph Winters
-
Interviewed by Fast Company, “Your bottled water could contain 130,000 microscopic pieces of plastic”, by Kristin Toussaint
-
Interviewed for NJ 101.5, “NJ researchers find disturbing level of tiny fragments in most bottled water”, by Dino Flammia
-
Interviewed for US Vogue, “Is there a Eco-Friendly Way to have Glitter in Our Makeup?” by Tamar Adler
-
Interviewed for Canadian Broadcasting Corporation radio show, Quirks and Quarks, for feature “Plastic pollution and disease — ‘Plasticosis’ is a new plague for wildlife”, by Sonya Buyting
- Guest for TVO Television show, The Agenda with Steve Paikin, “Are We Becoming Plastic People”, hosted by Steve Paikin and produced by Eric Bombicino
- Interviewed for Bon Appétit magazine, “How Much Microplastic Am I Eating? And Is There Any Way to Avoid It?” written by Alison Francis
- Interviewed for EHP Science Selection, “Breach of Security? Placental uptake of micro- and nanoplastic particles” by Silke Schmidt
- Interviewed for Radio Health Journal, “What You Should Know About Ingesting Microplastics”, produced by Kristen Farrah
- Interviewed for and quoted in The Guardian article “Plastic particle pass from mothers into fetuses, rat study shows”, written by Damian Carrington
- Guest for National Public Radio (NPR), WHYY radio show Radio Times, “What to know about plastics and our health”, hosted by Tracey Matisak and produced by Debbie Bilder
- Interviewed by People Magazine, Earth Day Issue, “What This Scientist Says You Need to Know About Hundreds of Thousands of Plastic Particles in Your Drinking Water (Exclusive)”,by Johnny Dodd
- Interviewed for US Vogue, “The Latest Thing to Optimize? Your Drinking Water”,by Tamar Adler
- Interviewed for NJ Spotlight News, estimates, study says”, by Briana Vannozzi
- Interviewed for MedicalNewsToday, “Massive number of plastic particles found in bottled water. Are they harmful to health?”, by Robby Berman
- Interviewed by NPR Here and Now, “Plastic bottles shed invisible nanoplastics, study finds”, by Deepa FernandeInterviewed for CBS Evening News with Norah O’Donnell, “Bottled water contains up to 100 times more plastic than previously estimated, new study says”, by Aliza Chasan
- Phoebe Stapleton, Ph.D., A.T.C. featured in the Plastic Soup Foundation series “Inhale, Exhale” in Netherlands – February, 2021 —WATCH SEGMENT
- Dr. Stapleton and her collaborators were featured in The Guardian’s story “Plastic particle pass from mothers into fetuses, rat study shows“.
- Phoebe Stapleton, Ph.D., A.T.C. featured in Consumer Reports: How to Eat Less Plastic. May 5, 2020
- Dr. Stapleton and her collaborators were awarded the 2019 Best Paper of the Year, published in Particle and Fibre Toxicology. Their manuscript, ‘Maternal Engineered Nanomaterial Inhalation During Gestation Alters the Fetal Transcriptome’, represents the best cutting-edge research published in the journal. The paper was selected by the journal’s Editorial Board and is scored by its citations, downloads, and impact in the year. This award-winning manuscript can be found here and more information about the journal Particle and Fibre Toxicology can be found here.
- Dr. Stapleton was quoted in TIME magazine article, “Should You Worry About Plastic Particles in Bottled Water?” (6/3/2019).
- Ms. Charlotte Love was awarded a 2019 Pfizer SOT Undergraduate Travel Award for her work in the Stapleton Lab during her 2018 SURF experience.
- Dr. Stapleton’s recently published study was highlighted in Rutgers Today (3/2019).
- Ms. Jeanine D’Errico was awarded a 2017 BMS Summer Internship within the Department of Drug Safety Evaluation worked alongside members of their Molecular Toxicology group.
Recent Publications
- Qian, N, Stapleton, P, Yan, B, Min, W. Reply to Materić: Appropriate blanks should avoid major contamination sources in the lab. Proc Natl Acad Sci U S A. 2024;121 (48):e2415874121. doi: 10.1073/pnas.2415874121. PubMed PMID:39546558 PubMed Central PMC11621815
- Moreno, GM, Brunson-Malone, T, Adams, S, Nguyen, C, Seymore, TN, Cary, CM, Polunas, M, Goedken, MJ, Stapleton, PA. Identification of micro- and nanoplastic particles in postnatal sprague-dawley rat offspring after maternal inhalation exposure throughout gestation. Sci Total Environ. 2024;951 :175350. doi: 10.1016/j.scitotenv.2024.175350. PubMed PMID:39117197 PubMed Central PMC11487574
- Cary, CM, Fournier, SB, Adams, S, Wang, X, Yurkow, EJ, Stapleton, PA. Single pulmonary nanopolystyrene exposure in late-stage pregnancy dysregulates maternal and fetal cardiovascular function. Toxicol Sci. 2024;199 (1):149-159. doi: 10.1093/toxsci/kfae019. PubMed PMID:38366927 PubMed Central PMC11057520
- Qian, N, Gao, X, Lang, X, Deng, H, Bratu, TM, Chen, Q, Stapleton, P, Yan, B, Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc Natl Acad Sci U S A. 2024;121 (3):e2300582121. doi: 10.1073/pnas.2300582121. PubMed PMID:38190543 PubMed Central PMC10801917
- Adams, S, Stapleton, PA. Nanoparticles at the maternal-fetal interface. Mol Cell Endocrinol. 2023;578 :112067. doi: 10.1016/j.mce.2023.112067. PubMed PMID:37689342 PubMed Central PMC10591848
- Stapleton, PA. The Application of Engineered Nanomaterials in Perinatal Therapeutics. Small. 2024;20 (41):e2303072. doi: 10.1002/smll.202303072. PubMed PMID:37438678 PubMed Central PMC10784409
- Cary, C, Stapleton, P. Determinants and mechanisms of inorganic nanoparticle translocation across mammalian biological barriers. Arch Toxicol. 2023;97 (8):2111-2131. doi: 10.1007/s00204-023-03528-x. PubMed PMID:37303009 PubMed Central PMC10540313
- Cary, CM, Seymore, TN, Singh, D, Vayas, KN, Goedken, MJ, Adams, S, Polunas, M, Sunil, VR, Laskin, DL, Demokritou, P et al.. Single inhalation exposure to polyamide micro and nanoplastic particles impairs vascular dilation without generating pulmonary inflammation in virgin female Sprague Dawley rats. Part Fibre Toxicol. 2023;20 (1):16. doi: 10.1186/s12989-023-00525-x. PubMed PMID:37088832 PubMed Central PMC10122824
- Cary, CM, DeLoid, GM, Yang, Z, Bitounis, D, Polunas, M, Goedken, MJ, Buckley, B, Cheatham, B, Stapleton, PA, Demokritou, P et al.. Ingested Polystyrene Nanospheres Translocate to Placenta and Fetal Tissues in Pregnant Rats: Potential Health Implications. Nanomaterials (Basel). 2023;13 (4):. doi: 10.3390/nano13040720. PubMed PMID:36839088 PubMed Central PMC9965230
- Bowdridge, EC, Thompson, J, Bourque, S, Stapleton, P. Editorial: Getting to the heart of developmental toxicities. Front Toxicol. 2023;5 :1138470. doi: 10.3389/ftox.2023.1138470. PubMed PMID:36726487 PubMed Central PMC9886310
- Engler-Chiurazzi EB, Stapleton PA, Stalnaker JJ, Rambo-Hernandez KE, Sarkar SN, Jun S, Quintana DD, Ren X, Hu Heng, Nurkiewicz TR, McBride CR, Yi J, Simpkins JW. Adult Behavioral Consequences of Prenatal Engineered Nanomaterial Exposure in Rodents. Journal of Toxicology and Environmental Health Part A. 2016; 79(11): 447-52. doi: 10.1080/15287394.2016.1164101.
- Stapleton PA. Gestational xenobiotic exposures: microvascular implications for the past, present, and future. Journal of Physiology. Apr; 594(8): 2161-73, 2016. (invited symposium review) doi: 10.1113/JP270581.
- Stapleton PA, Nichols CE, Yi J, McBride CR, Minarchick VC, Shepherd DL, Hollander JM, Nurkiewicz TR. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology. 2015 Nov;9(8): 941-51. doi: 10.3109/17435390.2014.984251.
- Nichols CE, Shepherd DL, Knuckles TL, Thapa D, Stricker JC, Stapleton PA, Minarchick VC, Alway SE, Nurkiewicz TR, Hollander JM. Cardiac and Mitochondrial Dysfunction Following Acute Pulmonary Exposure to Mountaintop Removal Mining Particulate Matter. American Journal of Physiology – Heart and Circulatory Physiology. Dec; 309(12): H2017-30, 2015. doi: 10.1152/ajpheart.00353.2015.
- Stapleton PA, McBride CR, Yi J, Nurkiewicz TR. Uterine microvascular sensitivity to nanomaterial inhalation: an in vivo assessment. Toxicology and Applied Pharmacology. Nov; 288(3): 420-8, 2015. doi: 10.1016/j.taap.2015.08.013
- Stapleton PA, Minarchick VC, Yi J, Engels K, McBride CR, Nurkiewicz TR. Maternal engineered nanomaterial exposure and fetal microvascular function: does the Barker hypothesis apply? Am J Obstet Gynecol. Sep; 209(3): 227.e1-227.e11, 2013. doi: 10.1016/j.ajog.2013.04.036.
- Stapleton PA, Minarchick VC, Cumpston AM, McKinney W, Chen BT, Sager TM, Frazer DG, Mercer RR, Scabilloni J, Andrew M, Castranova V, Nurkiewicz TR. Impairment of coronary arteriolar endothelium-dependent dilation after multiwalled-carbon nanotube inhalation: a time course study. IJMS. Oct; 13: 13781-13803, 2012. doi: 10.3390/ijms131113781.
- Stapleton PA, Minarchick V, Knuckles TL, Nurkiewicz TR. Xenobiotic Particle Exposure and Microvascular Endpoints: A Call to Arms. Microcirculation. Feb; 19(2):126-42, 2012. doi: 10.1111/j.1549-8719.2011.00137.x.
Copyright © 2021, Rutgers, The State University of New Jersey


